According to CDC (Centers for Disease Control and Prevention), COVID self-testing, which is also often referred to as “at-home testing,” is one of the strategies that helps to reduce the risk of COVID-19 transmissions.
You can perform these tests at home before going to school, work, or any social gathering to make sure that you are not infected at the moment. They are purchased over-the-counter from your pharmacy or retailer. They also are easy to use and are equally indicative regardless of your vaccination status or the presence or absence of COVID-19 symptoms.
This is the first of two articles aimed to address the most common questions about COVID self-testing and to provide the most current and relevant information about available at-home tests and their use.
.
.
How do COVID-19 At-Home tests work?
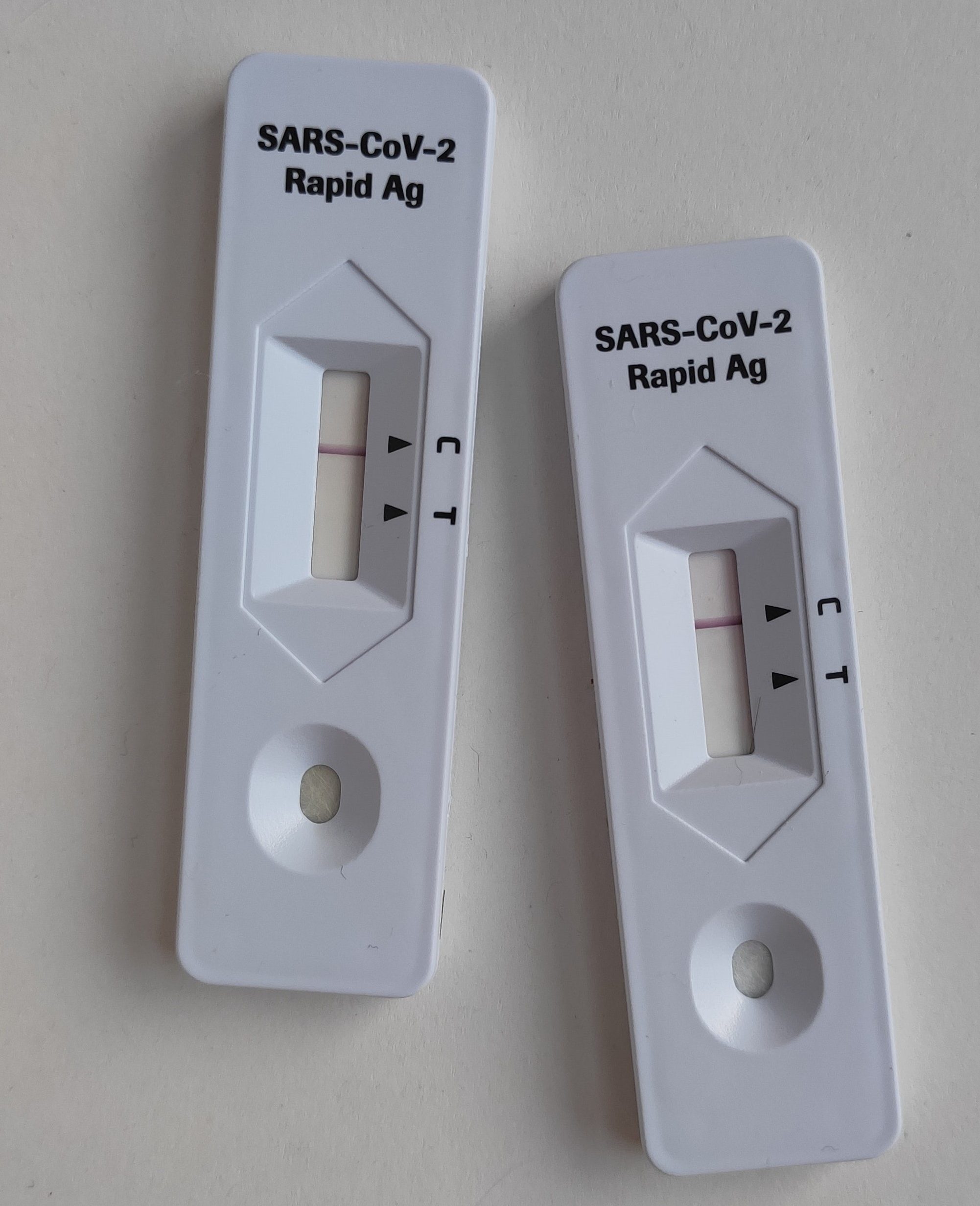
At-home tests come in a safe and convenient form of nasal swabs, which can be done anywhere. They can provide a result within 15-30 minutes. Because they are quick and convenient, these tests are also known as rapid tests.
All currently approved COVID self-tests are the “antigen tests” you hear about in the media. It means that they can detect the materials that a virus can leave behind, for example, the proteins. They don`t, however, recognize the virus`s actual generic material. Technically, it is like seeing animal fur on furniture and assuming a pet in the house.
.
What is the difference between a PCR and Antigen COVID test?
Both PCR and Antigen testing start with a sample from the patient or participant. It can be a nasal swab or saliva sample.
However, PCR testing aims to detect the virus`s DNA in the sample through a polymerase chain reaction technique. This kind of testing provides a more accurate and definitive result but requires special equipment and a skilled lab technician to run them properly.
Antigen tests use lab-made antibodies to search for antigens from the SARS-CoV-2 virus. Those are the substances that force our immune system to produce antibodies. The testing strip used in such tests binds to the antigen in a sample and indicates it with a colored stripe.
Antigen tests (i.e., rapid tests) are faster and easier to perform, but they are less sensitive and accurate than PCR testing. In other words, if the accuracy of PCR COVID tests approaches 100%, Antigen home testing, by comparison, identifies the infection in 72% of people with symptoms and 58% of people without symptoms.
How much do COVID At-home testing kits cost?
When COVID self-testing kits were first introduced to the general public, costs ranged between $25 and $50. Currently, several brands of Antigen tests are available for about $20. Costs are higher for PCP tests
Self-testing kits may likely follow a similar price pattern as facemasks, which skyrocketed in price at the beginning of the pandemic, only to come down significantly and be made available to individuals in need of them in healthcare clinics and public places.
According to the latest statements of President Biden`s Administration, Americans will get access to over 150 million free home-testing kits during the holiday season. The tests will be available through local libraries, schools, and healthcare facilities.
But before you head to your local library, it is recommended that participants call ahead because these facilities have limited storage space, and products are delivered in batch amounts. If the library doesn’t have a kit available and you cannot get to a facility to be tested, rapid kits can still be purchased from a retailer, such as your local pharmacy.
In addition, retail giants such as Walmart and Amazon announced the possible launch of low-cost COVID self-testing kits for travelers during the holiday season. You can purchase a single kit or a multipack containing several kits so you can use them as needed for yourself or to give to family members.
In the next article we will discuss the most popular at-home tests and how to use them.
.
Recent Comments